After months of speculation and rumours, Doctor Who fans finally know who will replace Jodie Whittaker as the new Time Lord: the latest and 14th formal incarnation of the titular Doctor Who will be the 29-year-old actor Ncuti Gatwa.
Every time this character has regenerated over the past six decades their cells have been rewritten, their wounds repaired, and their severed limbs regrown.
The dramatic reasoning behind regeneration is explained by Glyn Morgan, lead curator of the forthcoming Science Museum blockbuster exhibition, Science Fiction: Voyage to the Edge of Imagination:
“The Doctor’s regeneration (although they called it ‘renewal’ at the time) first appeared in 1966 as a practical method of replacing the first Doctor, an aging William Hartnell, with the sprightlier (although still mid-forties) Patrick Troughton whilst maintaining narrative continuity,” says Morgan. “The regeneration sequence was also inspired by the experience of an LSD trip and must have been both a shock and quite scary for fans of the show at the time.”
But, although the transformation of one person into another sounds a little far-fetched, experiments on creatures such as fruit flies have shown how genetic changes can alter appearance.
And there are plenty of clues in the animal kingdom, in creatures such as salamanders, starfish, crabs and lizards, that basic regeneration may be possible, comments Sir Jim Smith, emeritus scientist at The Francis Crick Institute, in London.
The 10th Doctor, played by David Tennant, had his right hand severed which later regenerated into a whole Doctor, and in the same way the planarian Schmidtea mediterranea, a type of flatworm, can regrow an entire animal from tiny tissue fragments as minuscule as 1/279th of the animal.
“This illustrates a fundamental principle about regeneration, which is that of pattern formation,” comments Sir Jim. “The great British developmental biologist Lewis Wolpert used to emphasise in lectures that cells on the amputated stump know their position along the axis of the limb, and know to regenerate in the right direction. Otherwise, when you cut off a newt’s limb, if regeneration went in the wrong direction, it might well regenerate a whole new newt, holding hands, as it were, with the old one.”
The recipe to build a body is held in the form of DNA in cells and scientists are beginning to uncover the genetic ingredients of regeneration. As one example, a study published recently by the Sánchez Alvarado Lab at the Stowers Institute for Medical Research in Kansas City studied how genes were used, or ‘expressed’, at the single-cell level, across all of the different cell types of a regenerating animal.
The study, led by Dr Blair Benham-Pyle, showed whole-body regeneration involves changes in cells from all three basic ‘germ’ layers that make up the body (muscle, epidermis, and intestine), for example.
Even a whole head can regenerate, according to a study of Hydra, a freshwater creature that looks like something out of Doctor Who, a floating tube with a grasping appendage at one end and a cluster of tentacles at the other, albeit only an inch or so long.
Recently, a study mapped out how Hydra can regenerate their own heads by changing the way that their genes are turned on and off. In the study published in Genome Biology and Evolution, Aide Macias-Muñoz of the University of California, Irvine, and colleagues first identified more than 27,000 genetic elements that play a role. This feat makes the creature immortal, just like the Doctor.
By comparison with Hydra, a normal person only has limited regenerative abilities, notably refreshing blood, a degree of repair to ear lobes and fingertips, along with the liver. It could be that our distant ancestors once had the ability to regenerate, but it is now dormant. The issue of why and how regenerative ability was lost ‘is a really interesting question,” said Sir Jim.
A hint that this ability could be revived came earlier this year when Nirosha Murugan and David Kaplan at Tufts University and colleagues at Harvard University’s Wyss Institute reported how they had enabled frogs to regrow a functional, nearly complete limb.

In experiments on adult frogs, which are naturally unable to regenerate limbs, the researchers were able to trigger regrowth of a lost leg using a five-drug cocktail applied in a silicone wearable bioreactor dome that seals in the cocktail over the stump for just 24 hours.
That brief treatment sets in motion an 18-month period of regrowth that restored a functional leg, a little slower than Time Lord regeneration which normally seems to resolve itself within a 45-minute episode. “One of the things about development is that it is really hard to speed up,” said Sir Jim. “Shortcuts might be possible, but they might well be risky.”
Another aspect of regeneration is changing the age of tissues and this feat has now been achieved by a team led by Wolf Reik at The Babraham Institute in Cambridge, which has achieved cellular time travel, rewinding the cellular biological clock by around 30 years according to molecular measures, significantly longer than previous reprogramming methods.
The partly rejuvenated cells that resulted from the ‘time jump’ showed signs of behaving more like youthful cells in experiments simulating a skin wound, they found. Though at an early stage, the work could eventually have implications for regenerative medicine.
Regenerative biology aims to repair or replace cells including old ones, notably with stem cells, parent cells that can form other types. One of the most important tools in regenerative biology is our ability to create ‘induced’ stem cells, where the ‘marks’ that control how the genetic code is used to give a cell an identity, whether brain or muscle, are wiped clean, first developed in 2006 by Shinya Yamanaka.
The clever part of the Babraham method was that they wanted to avoid the problem of entirely erasing cell identity and achieved this by halting reprogramming part way through the process. This allowed researchers to find the precise balance between reprogramming cells, making them biologically younger, while still being able to regain their specialised cell function.
Science can also shed light on other features of regeneration. Among hardcore fans, it is thought that Earth’s favourite Gallifreyan can only have 12 regenerations. ‘Perhaps this Whovian mythology is a reference to the Hayflick limit,’ said Sir Jim.
As demonstrated by Leonard Hayflick in 1961, at the Wistar Institute in Philadelphia, Pennsylvania, human cells have a limited replicative lifespan, with older cells reaching this limit of around 50 cell divisions sooner than younger cells.
This “Hayflick limit” is directly related to the number of unique DNA repeats found at the ends of the genetic material-bearing chromosomes, part of structures, called “telomeres,” which protect the ends of chromosomes, the bundles of DNA in our cells.
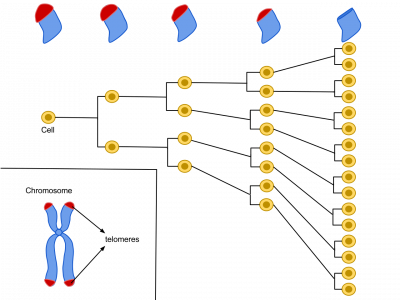
‘’But this limit is more to do with lifespan than regeneration, and the question of longevity has recently become particularly interesting with the observation that the rate at which mutations build up in an animal is inversely related to its lifespan” adds Sir Jim.
Glyn Morgan, curator of Science Fiction, concludes: “Over the years, the regeneration of the Doctor has allowed successive generations of producers to revamp and refresh the show, taking it in different directions and opening up new possibilities and plotlines, it is probably the most important part of the beloved programme’s near 60-year longevity.”
Science Fiction: Voyage to the Edge of Imagination is open at the Science Museum until 20 August 2023. Book your tickets now
