See IVF: 6 Million Babies Later in Who Am I? until 25 November 2018.
Perhaps one of the most fascinating questions in Biology is how a single cell, the fertilized egg, gives rise to the complex myriad of cell and tissue types present in the adult organism, in an organized and timely manner.
By the late 1400s, Leonardo Da Vinci had already dissected a human fetus and concluded that embryos change in size and shape during development. But if we are to understand how these changes take place, and how they go awry in cases of miscarriage and pregnancy loss, we need to study human embryos.
Many centuries had to pass before Bob Edwards achieved a revolutionary scientific milestone: the fertilization and in vitro maturation of a human egg. This achievement opened the doors for the treatment of infertile couples, but it also gave scientists the opportunity to look at human embryos developing in vitro for the first time in history.
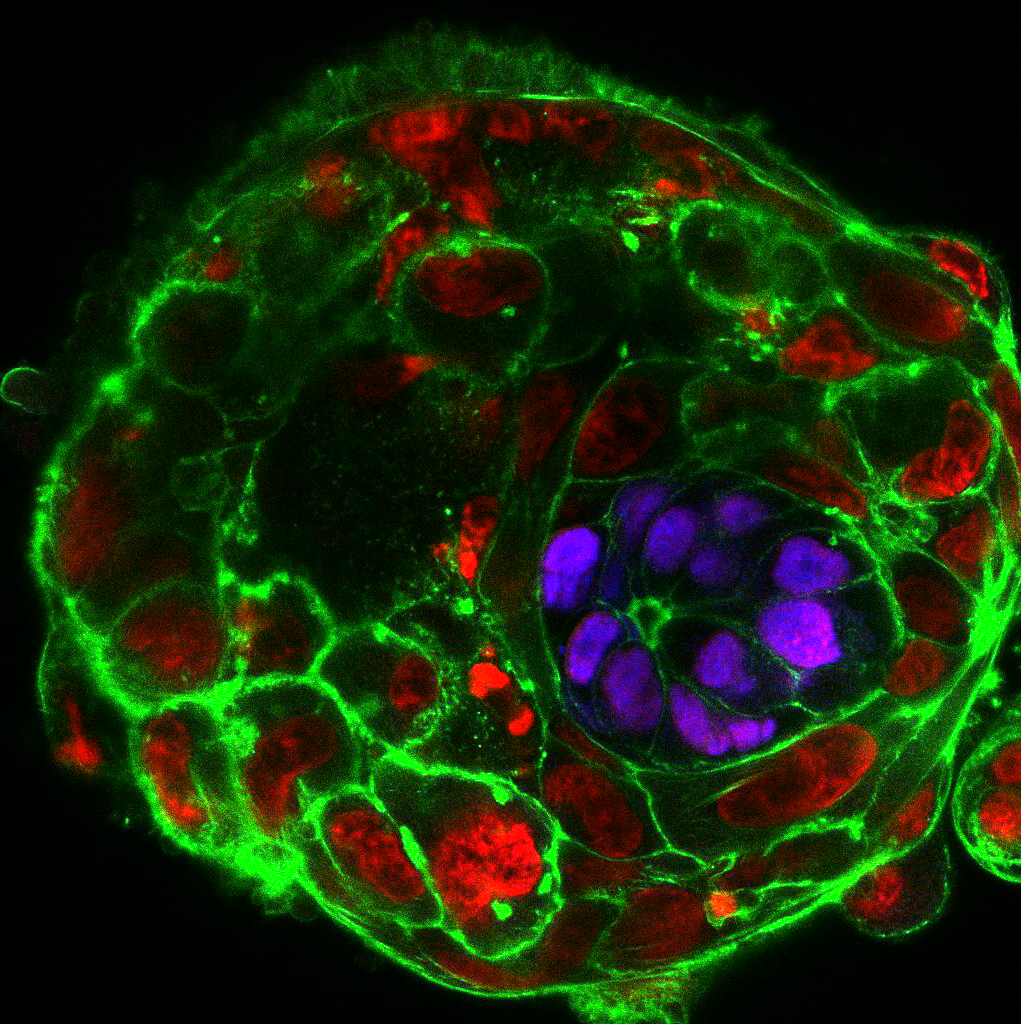
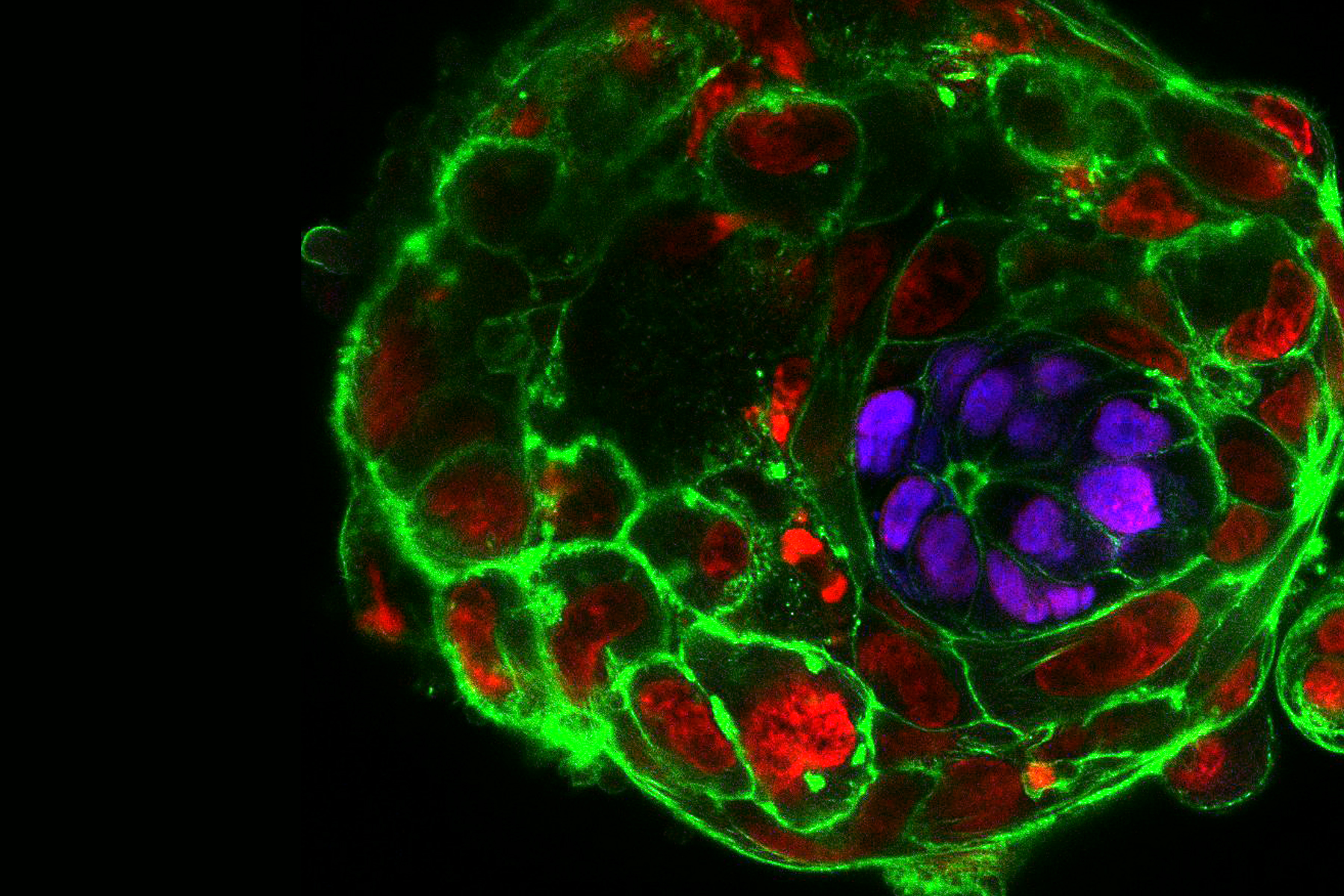
Upon fertilization, the human egg undergoes a series of divisions and changes in shape, culminating with the formation of an embryo, called the blastocyst, composed of approximately 200-300 cells on the 5th day of development. An embryo that reaches this stage is ready to implant into the maternal uterus, and, therefore, in IVF clinics embryos are transferred to the women’s uterus on day 5. But what happens to the embryo after implantation? 30% of human embryos fail to develop normally during the first post-implantation week. Yet, since the embryos are placed in the uterus, we cannot see them or study them to provide an answer. Could we find a way around it?
The starting point of our research was the hypothesis that human embryos could develop beyond implantation without the maternal uterus. At first this may seem unrealistic, but we already knew that mouse embryos could develop in a petri dish beyond the implantation point. And if mouse embryos could do it, why wouldn’t human embryos?
To perform these experiments we had to first seek approval from the Human Fertilization and Embryology Authority, as well as an independent ethical approval from a Research Ethics Committee. This legal framework allowed us to use surplus human embryos from IVF treatments, which were donated by couples that received informed consent.

In our daily routine we collect, manipulate and observe mouse embryos. Watching how they develop in perfect harmony is enchanting, but it definitely does not compare to the thrill and excitement that we experienced when we cultured the first human embryos beyond day 7, day 8, day 9… and all the way up to day 13. Not only did the embryos survive and grow, they also formed various post-implantation structures. We observed the formation of the amniotic cavity, which protects the developing embryo from damage; the appearance of specialized cells that build the placenta and communicate with the mother; and the formation of a rudimentary yolk sac, which provides nutrients.
But the excitement has just begun! We are now using this new method to understand why some embryos develop well and some others fail. We are particularly interested in understanding what happens when cells in the embryo do not have the right number of chromosomes, a phenomenon known as aneuploidy, as this is a major limitation for human reproduction. By studying human embryos that would otherwise be discarded we want to understand why so many human pregnancies fail, so we can hopefully conceive new strategies to fight infertility.
See IVF: 6 Million Babies Later in Who Am I? until 25 November 2018. Find out more.