With this year being the International Year of the Periodic Table, it’s an exciting time to be a chemist. People around the world are celebrating the birth of one of the most elegant and impressive organising principles of nature. The building blocks that make up everything we know are displayed through Mendeleev’s iconic chart that graces the walls of almost every chemistry classroom, lecture hall and textbook.
But the periodic table that we all know now is not static, fixed or unchangeable and is far from perfect. The current form is known to have its flaws. For example, the block design that arranges the elements by columns and rows breaks the continuity between the elements as you move from one period to the next. Another criticism is the fact that the two rows below the main table, called the lanthanides and actinides, appear as an awkward addition.
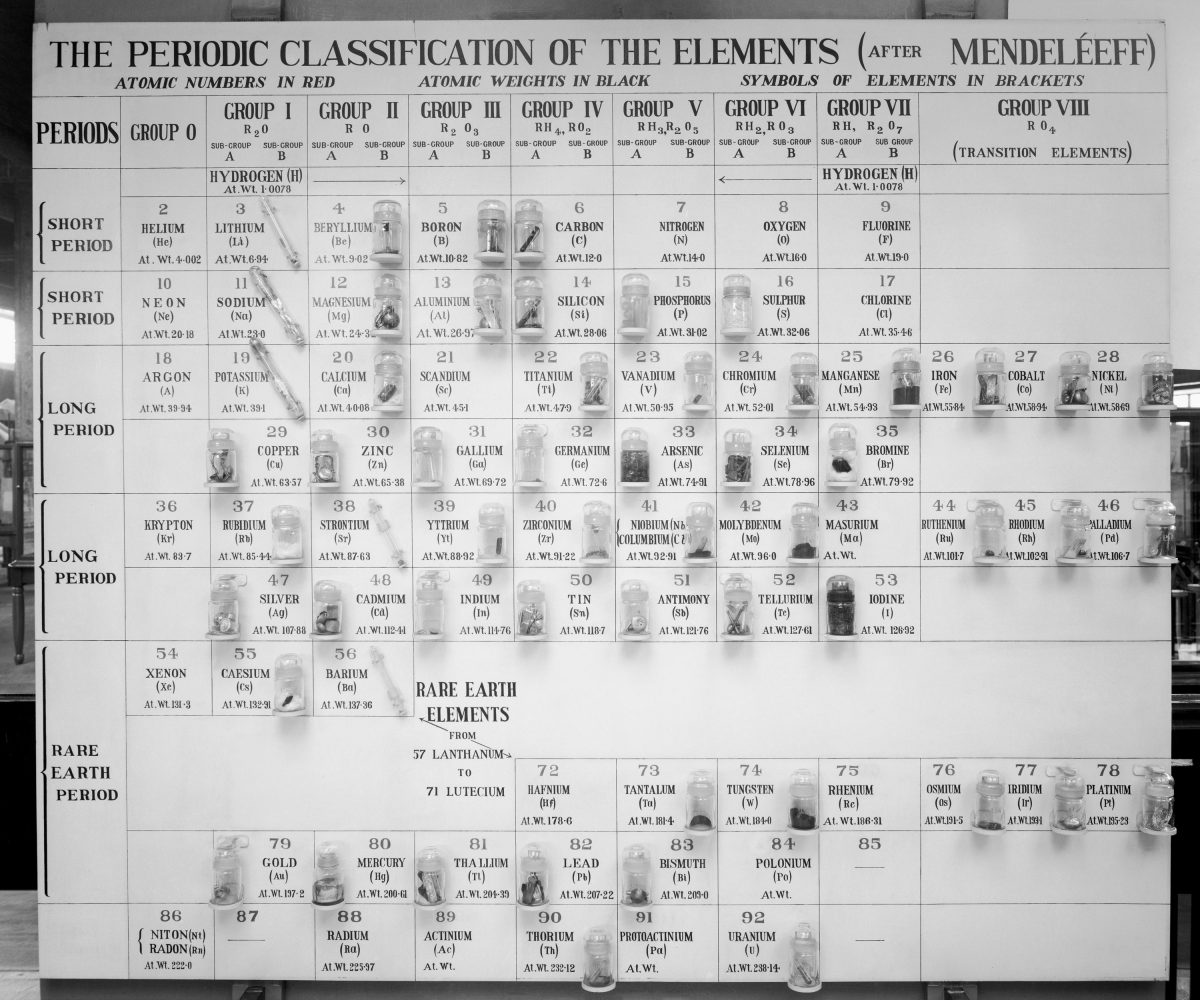
In some ways, it falls short in demonstrating ‘periodicity’ – the idea of recurring trends between elements when arranged in order of increasing atomic number.
Because of these flaws, scientists and educators have strived to make new and original representations of the periodic system using a range of forms, from spirals and helices to spheres and tetrahedrons. There’s even a database (see here), that contains hundreds of different versions.
For my dissertation project at University College London, I’ve been diving into the Science Museum Group’s collection, tracing the history of alternative versions of the periodic table.

I’m interested in how three-dimensional models like this Spiral Periodic Table allow us to learn about the complex relationships between the elements.
Supported by poles and twisting around itself in a snake-like manner, this object is one of many weird and interesting forms of the periodic table. It was built at the Royal Military College of Science in 1963. The Science Museum asked for this model to be made for them to display in their new chemistry gallery after the original model was seen at an exhibition held by the Physical Society.

This is a very dynamic and interesting way to depict the elements. Because it is displayed as a spiral, there is a continuity between elements that is not achieved with the two-dimensional table.
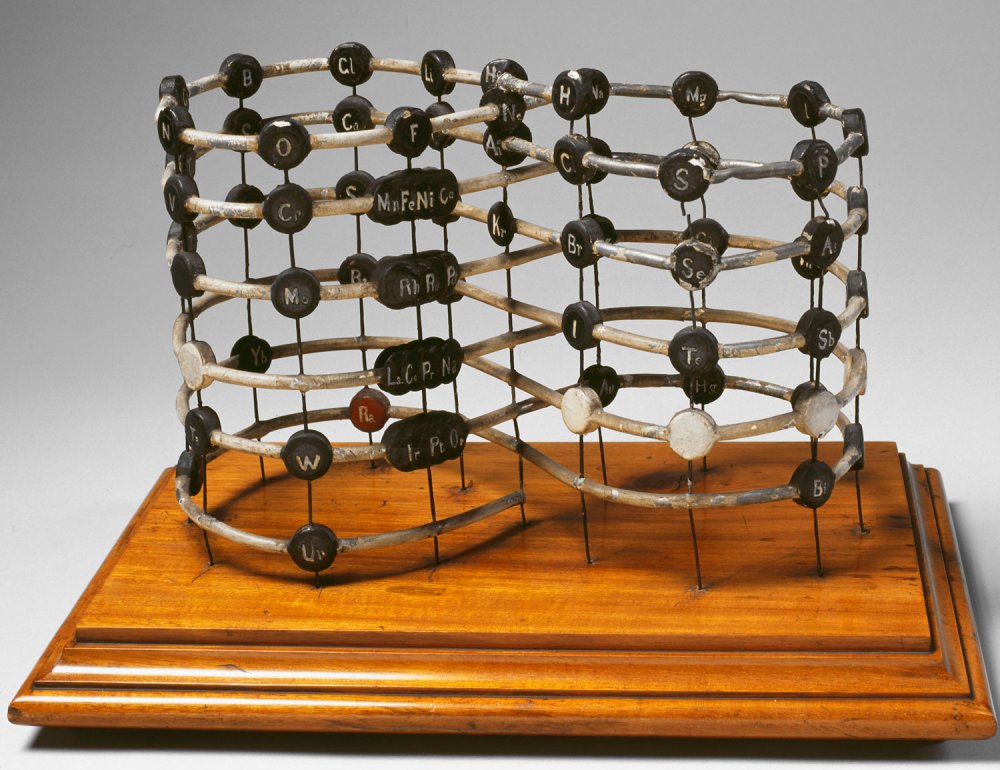
This model, housed in a wooden cabinet, is a physical realisation of arguably one of the first periodic systems to ever be created. It was made by the Science Museum’s workshops in 1925, based on the periodic spiral of Alexandre de Chancourtois, a French geologist, who conceived it in 1862.

The elements are plotted in order of atomic weight on a line that twists around a cylinder at an angle of 45 degrees. It comes with a dial that allows you to rotate the cylinder inside.
It is possible that because he was a geologist, de Chancourtois was inspired to demonstrate periodicity in a drum and stick model similar to those used to measure earthquakes.
This ‘pretzel-shaped’ model, was made by Sir William Crookes in 1888. As well as showing the relationships between chemicals, he wished to also show their evolution, which originated from what he called ‘primal matter’ in the stars. When cooled, it began to form the elements starting with the lightest, hydrogen, at the top of his table, down to uranium, the heaviest at the bottom.
More recent versions have been created for teaching purposes. One is a tree-like structure created by the Canadian chemist Ferdinand Dufour. By looking downwards from above, you can see what is known as ‘secondary periodicity’, which are hidden relationships between elements that might be further away from each other than in the traditional periodic table.
Another, is the Periodic Round Table, created by Gary Katz. It exhibits a symmetry and regularity that the other tables do not provide. Both models can be taken apart and reassembled, providing opportunities for tactile learning.
But why visualise the periodic table in three dimensions?
With 3D tables, we can touch them, walk around them, and in some cases even build them. They provide us with new and dynamic ways to learn about the elements, see new relationships between them, and have the potential to inspire curiosity.
This is not at all to say that we should abandon the current periodic table, but perhaps we should embrace more dynamic forms of the periodic table in our labs and classrooms to better learn about and investigate the complexities behind chemistry.
Discover more about the evolution of the periodic table here.
We are interested in collecting items related to the International Year of the Periodic Table (from home-made periodic tables to printed ephemera), preserving these as part of the collection to record worldwide celebrations in 2019.
Delve into interesting stories of how chemistry affects the world around you in our online series.
